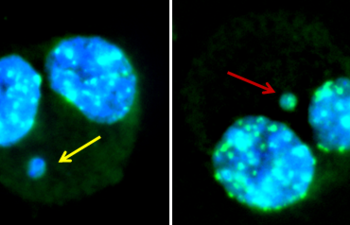
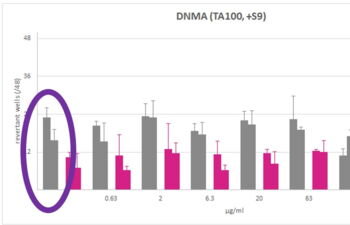
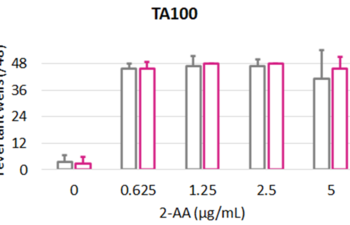
Products, Kits and Service Analytics for the detection of mutagenic, genotoxic, endocrine active molecules as well as skin models for absorption studies.
We are competent – We care for more than 200 customers worldwide in a variety of sectors (chemical, agrochemical, pharmaceutical and cosmetic industries as well as in the field of environmental protection). Xenometrix offers ready-to-use kits, individual bacterial and yeast strains, rat liver S9, reagents and service analytics optimized for studies in the fields of mutagenicity, genotoxicity, endocrine disruption or skin permeation. Xenometrix collaborates with a network of expert partners for service analytics for in vitro toxicity, ecotoxicology, biodegradability, environmental fate, analytical chemistry, and skin absorption.
We are SME – Being a SME, a lean management allows to be flexible and fast in communicating and making decisions. With a troubleshooting, customer-oriented mentality we have continuously grown in terms of knowledge, experience and competence for more than 25 years.
We are innovative – We constantly develop and improve products together with customers and suppliers and collaborating with world-renowned experts.
We are quality – Our competent employees are working flexibly and efficiently. Their maxim is customer oriented service and support. As a Swiss company quality has first priority, we produce and distribute exclusively high quality products. Xenometrix is certified for ISO9001(2015), ISO 13485 (in development).
We are responsible – As a scientific enterprise, we continuously reduce our environmental emissions by the means of responsible management of resources and energy.
We are future – Since 1995 we found ourselves fascinated by scientific findings and new technologies again and again. They challenge us daily, mobilize our energies and bring us satisfaction. We are ready for any future, new challenges.

 Navigation
Navigation